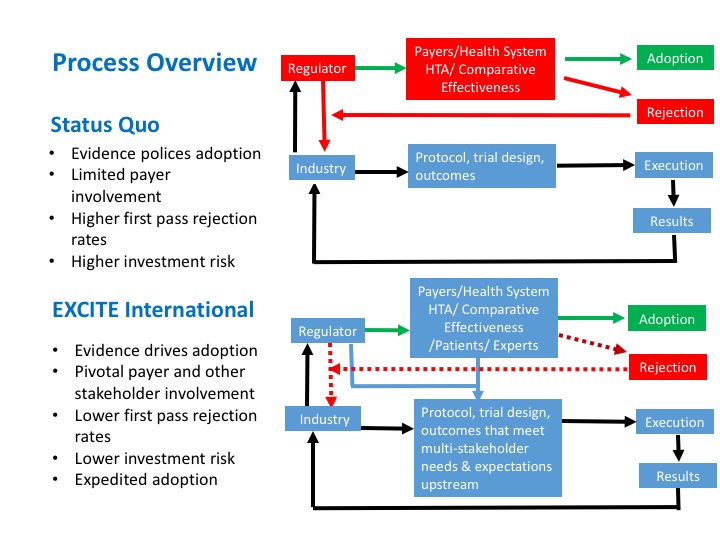
With EXCITE International’s collaborative approach, customized clinical trial protocols are designed with input from international payers, health systems, the scientific community, as well as expert end-users, patients, and other relevant key stakeholders.
With EXCITE International’s collaborative approach, customized clinical trial protocols are designed with input from multiple stakeholders representing the perspectives and expectations of regulators, payers, health systems, methodology and evidence based scientists, usability and human factors experts, safety and quality assurance experts, key opinion leaders and patients among our international partners. This all occurs at the point of consideration for studies to satisfy the expectations of regulators (“pre-market”) so that clinical trials harmonize regulatory and coverage determinations through a single process early on. This improves the chances of successful regulatory approval and first-pass coverage decisions downstream.
This unique international collaborative approach recognizes the importance of engaging key stakeholders early on to improve the likelihood of global adoption. It provides evidence needed to support adoption and health planning based on demonstrating that the technology improves the net health outcome for the indicated patient population. In so doing, this approach reduces the need for additional studies at the point of decision-making regarding coverage/payment and adoption and de-risks the existing fragmented pathway EXCITE International was designed to replace.
EXCITE International then develops and executes a protocol which is intended to meet the evidence needs of the key pre-and post-market decision-makers.
Our world-class methodological centres and clinical trial networks are used to implement the inclusive protocol to collect, analyze, and synthesize data for both regulatory and adoption purposes – our benchmark for success. The EXCITE International network collaborates with their local key stakeholders to select, evaluate, and adopt impactful technologies through their role as an EXCITE International partner. Oversight of each clinical trial is provided by designated credible Clinical Research Organizations and health system clinical trial networks.
Through the synchronous involvement of multiple stakeholders in EXCITE member countries, EXCITE International accelerates the pathway of high-impact technologies to patients and health systems worldwide.
See Also: